For many applications, it is necessary to bring two different material phases intimately together, such as dirt and water in surface cleaning, air, and water in foaming, organic chemical, and water in medicine and oil and water to form emulsions. However, such combinations are chemically opposite in nature and do not mix. There is a kind of strain between their phase boundaries, as seen between surfaces of water and oil. This strain is referred to as tension, more precisely, "interfacial tension" in case of Liquid-liquid and liquid-solid phases and surface tension in case of Liquid – Gas phases.
Some molecules are active at the "surface" or "interface" of two non-mixing materials, and they help reduce the strain between the two phases. These are Surface Active Agents, and the term used is "Surfactants." The surfactant molecules consist of two parts (Fig.1). One part of the molecule likes to be associated with water or "Water Loving Part," and the other part stays away from water into the oil the "Oil Loving Part." This unique structure of surfactant molecules helps reduce the interfacial tension between two different phases, thus keeping the two phases together for a more extended period.
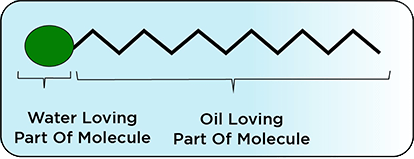 Fig.1
Fig.1
Structure of Surfactant
Cleaning is the process of removing dirt from the surface. Surfactants are generally used to clean surfaces. The composition of dirt could be mud, oil, color, sweat, sebum, etc. which could be partly water-soluble and partly water-insoluble. The water-soluble part of the dirt is removed from the surface easily using water but not the insoluble part. This difficulty arises due to the non-compatibility of the insoluble part of the dirt with water. If the surfactant is added in cleaning water, it reduces the "Interfacial Tension" between water and insoluble dirt by adsorbing at their interfaces. A Surfactant molecule acts as a mediator between dissimilar boundaries, insoluble dirt, and water. That enables the removal of dirt pieces under scrubbing action from the surface.
The surfactants have another interesting property exhibited in the water bulk. When present in sufficient concentration, they form aggregates in water called Micelles. Micelles are surfactant molecules that get oriented in a way that its "Oil Loving Part" (Fig. 1) forms a "Water Hating Core," and is shielded by "Water Loving Part" facing the water (Fig. 2). This "Water Hating Core" solubilizes water-insoluble dirt pieces removed during the scrubbing action in the cleaning process, overall stabilizing the system.
Surfactants, therefore, acts in two ways while cleaning the surfaces A) By bringing two different phases together to help remove the dirt and B) By removing dirt from the surface and solubilizing the dirt fragments in the core of the Micelle.
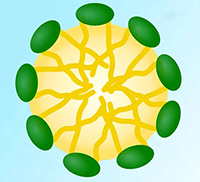 Fig. 2
Fig. 2
Water insoluble dirt inside Micelle
Besides cleaning, surfactants play a great role in various applications of our day to day use. They are used in a number of products in home care and personal care industry. In these applications, the formulation scientists exploit the unique structural property of surfactant molecules for homogenizing and emulsifying different dissimilar phases and to stabilize the formulation for giving greater shelf life to the products.